Research in the Herz group explores the fundamental science and applications of semiconducting materials and nanostructures ranging from organic molecules and solids, III-V inorganic semiconductors & nanostructures, to hybrid systems such as sensitized metal oxides and organic-inorganic perovskites. Current work focuses on common themes such as molecular self-assembly, energy and charge transfer, bio-mimetic light-harvesting, nanoscale electronic phenomena and interfacial effects. The group has leading expertize in femtosecond spectroscopic techniques, and applies this to unravel the ultrafast dynamics of excitations in a diverse range of semiconductor and nanostructures. Close collaborations exist with device physicists, theoretical researchers, synthetic chemists and materials scientists in order to advance the development of these novel materials for applications, such as light-emitting devices and next-generation photovoltaic cells.
|
New organic-inorganic metal halide perovskite materials emerged
recently as active materials in photovoltaic cells exceeding power
conversion efficiencies of 26%. Our group has been at the forefront
of developing an understanding of the fundamental processes that
underpin the outstanding performance of these materials. We investigate a wide range of topics in this area, including the
fundamentals of electron-phonon coupling, charge-carrier mobility
and recombination, light emission, photon reabsorption, atomic microstructure and ion migration. These studies
feed directly into collaborative efforts aimed at addressing remaining
challenges in the creation of commercially available perovskite solar
cells, e.g. stability, band-gap tunability, halide segregation, lead-free perovskites,
trap-free materials, material morphology control and alternative device structures.
|
|
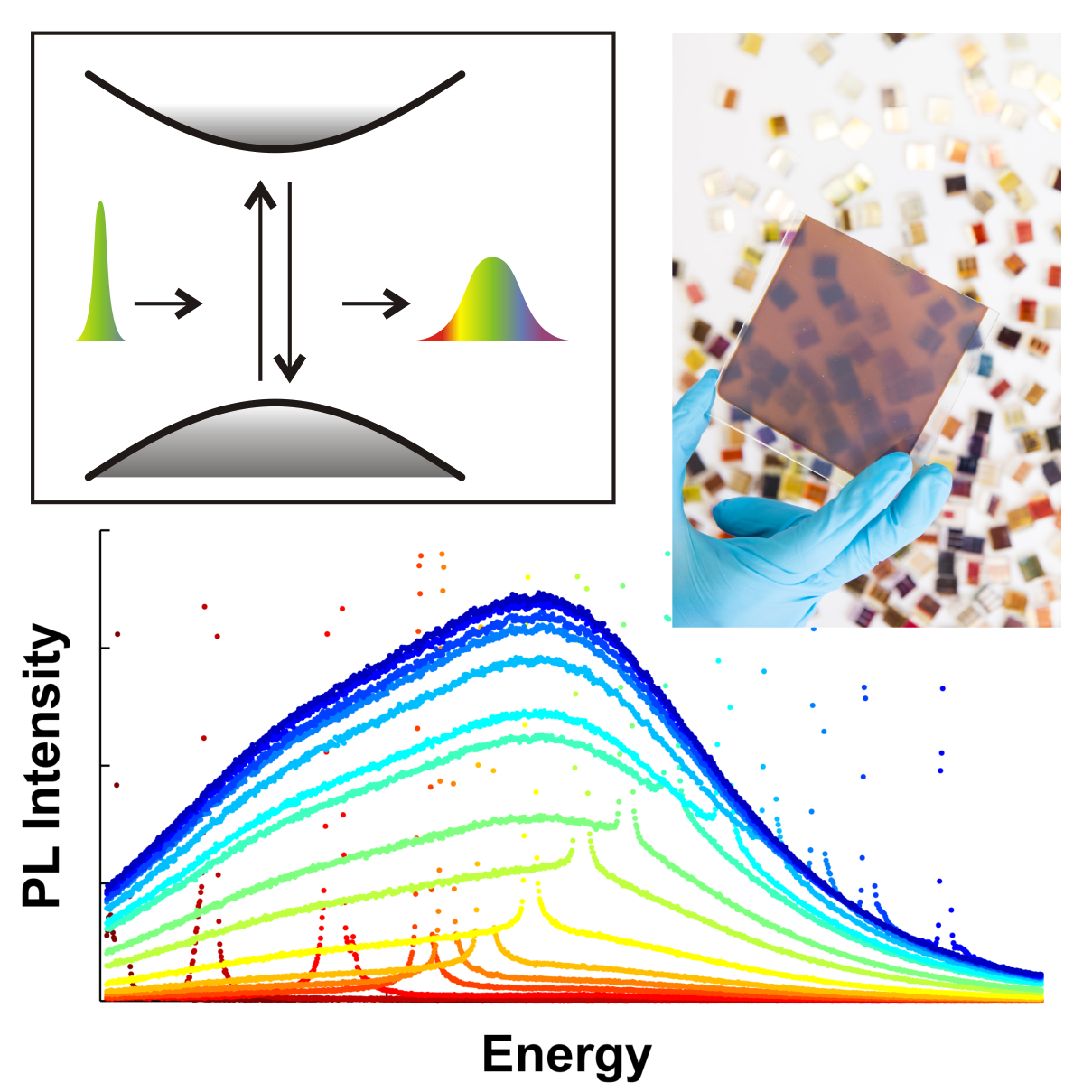
Low-dimensional halide perovskites, including nanocrystals and
two-dimensional layered structures, offer a multitude of device
applications, including efficient light-emitting devices and
photovoltaic cells. We explore the fascinating physics displayed by such systems of lower
dimensionality, examining e.g. how electronic quantum confinement affects exciton formation dynamics, charge-carrier
transfer and transport modes, and optoelectronic properties.
See e.g.: Adv. Func. Mater. 33 (2023), p. 2300363. ACS Energy Letters 8 (2023), p. 2543. Adv. Func. Mater. 30 (2020), p. 1909904. Nature Materials 19 (2020), p. 1201. Nano Lett. 19 (2019), p. 3953. |
|
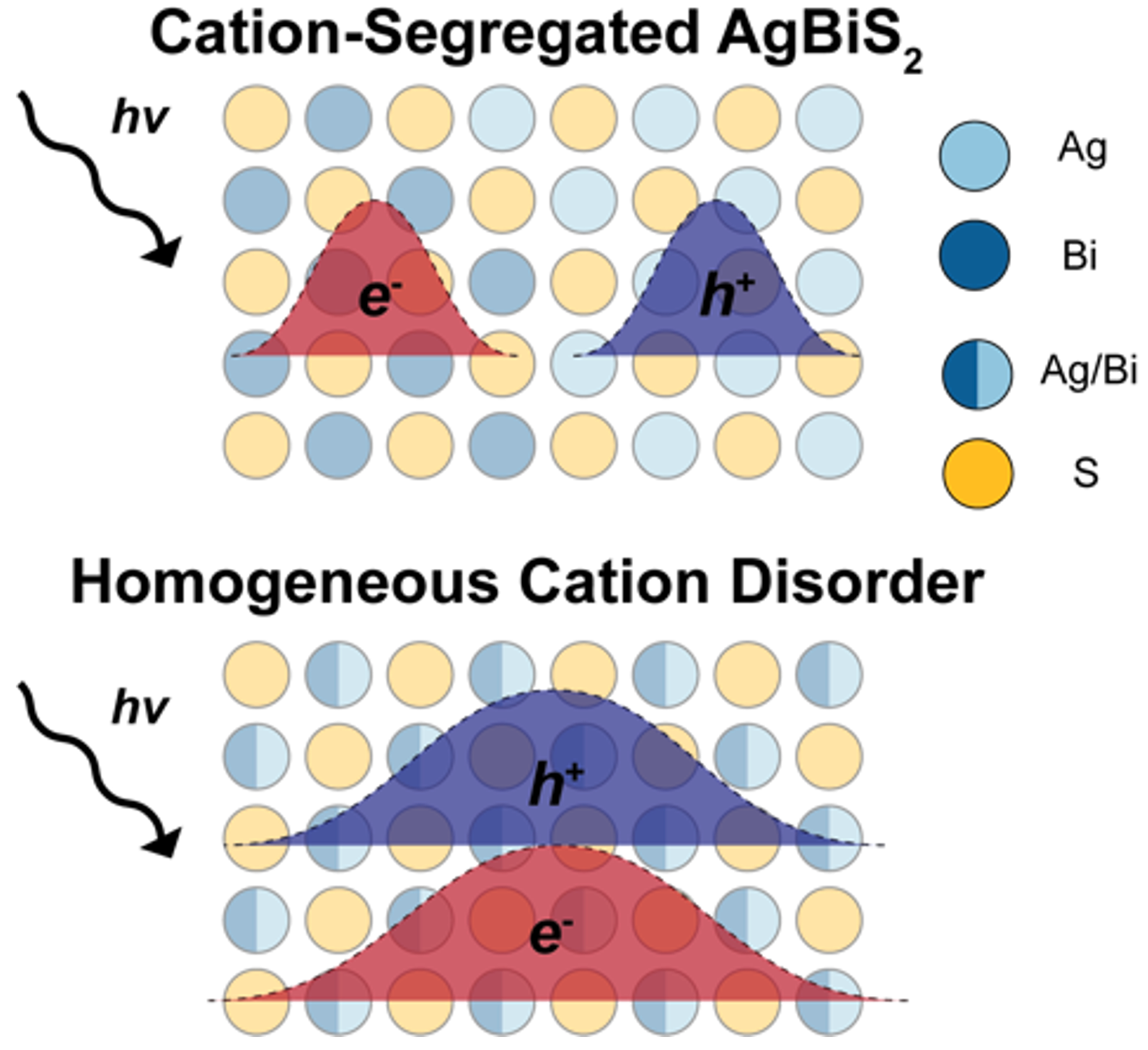
The stellar performance of lead halide perovskites has inspired the
search for new, related semiconductors with nontoxic inorganic
ingredients and high long-term stability. Our group investigates a
wide range of new chalcogenide, metal halide, and bisimide
semiconductors in this space, including Cs2AgBiBr6
Cs2AgSbBr6 and their alloys Cs2AgSbxBi1-xBr6, AgBiS2 and AgBiNa2 nanocrystals,
Ag3SI, BiOI, (4FPEA)4AgBiX8 (X = Cl, Br, I) layered materials, and
alloys in the CuI-AgI-BiI3 phase space, such as
Cu2AgBiI6. We investigate factors currently limiting the photovoltaic
performance of these materials, unravel the origin and impact of
ultrafast charge-carrier localisation effects, electronic and
structural dimensionality, alloying and cation disorder, and
bandstructure tuning.
|
|
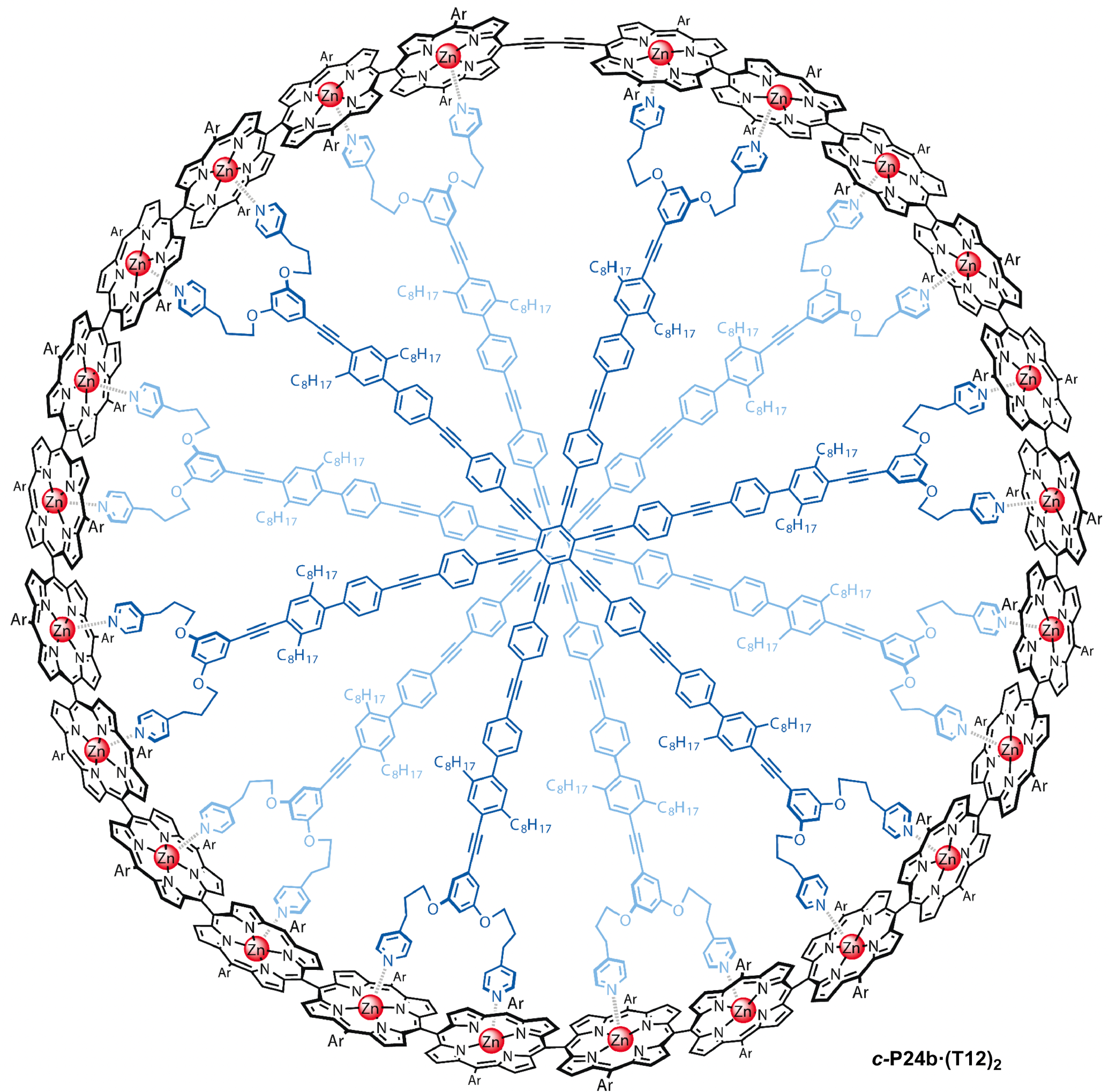
Natural evolution very early on solved the problem of how to capture
light efficiently and use it to initiate the primary electron
transfer reactions of photosynthesis in a surprisingly efficient
manner. We explore synthetic molecules that mimic such biological
light-harvesting complexes, but may ultimately allow high-efficiency conversion
of solar light into energy in the form of electrical current, rather
than biological mass. We examine the dynamics of photoexcitations in
Zn-porphyrin nanorings and nanostructures, revealing
electronic delocalization effects and the ultrafast
transfer of energy in multiple chromophoresforming part of nanostructured complexes.
See e.g.: Nature Chemistry, 14 (2022), p. 1436. J. Am. Chem. Soc. 141 (2019), p. 7965. J. Am. Chem. Soc. 140 (2018), p. 5352. ACS Nano 10 (2016), p.5933. J. Phys. Chem. Lett. 7 (2016), p. 332. J. Am. Chem. Soc. 137 (2015), p. 14256. Chemical Science 6 (2015), p. 181. J. Phys. Chem. Lett. 5 (2014), p. 4356. J. Am. Chem. Soc. 136 (2014), p. 8217. |
|
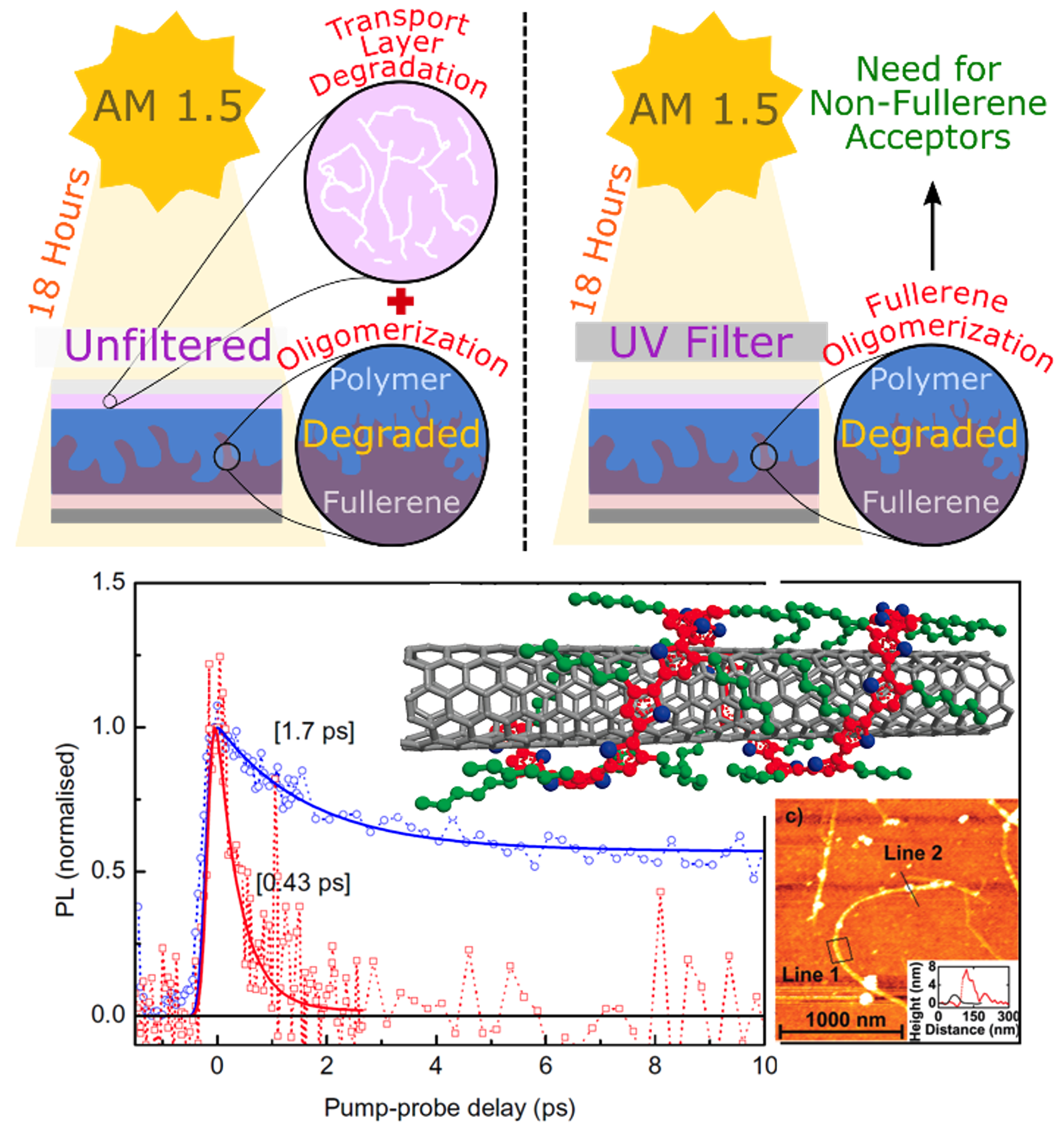
Organic pi-conjugated molecular materials offer another solution
towards efficient light-harveting and light-emitting
devices. The Herz group investigates ultrafast excitation
processes in a wide variety of organic (carbon-based) semiconductors
including conjugated polymers, small molecules, and carbon
nanotubes. We explore blend materials for OPV comprising electron and hole-transporting materials at whose
interface charges may dissociate, charge-extraction layers for
metal-halide perovskite solar cells, and LSCs which are cheap plastic lightguides containing
fluorophores that absorb light and channel it to the edge where a highly efficient PV cell is placed.
The Herz Group explores central aspects such as energy transfer, polarization
switching, electronic delocalization and lattice relaxation in organic semiconductors, and the impact of
morphology and intermediate charge-transfer states on charge generation in blends of electron and hole transporters.
See e.g.: Nature Communications, 11 (2020), p. 5525. J. Phys. Chem. Lett. 10 (2019), p. 1729. ACS Appl. Mater. Interfaces, 11 (2019), p. 21543 Nature Communications, 8 (2017), p. 15953. J. Phys. Chem. Lett., 6 (2015), p. 1170. Proc. Natl. Acad. Sci. U.S.A., 112 (2015), p. 7656. Adv. Optical Mater., 2 (2014), p. 687. J. Phys. Chem. C, 118 (2014), p. 17351. ACS Nano, 6 (2012), p. 6058. Nano Lett., 11 (2011), p. 66. J. Phys. Chem. Lett., 1 (2010), p. 2788. Phys. Rev. B, 78 (2008), p. 115321 |
|
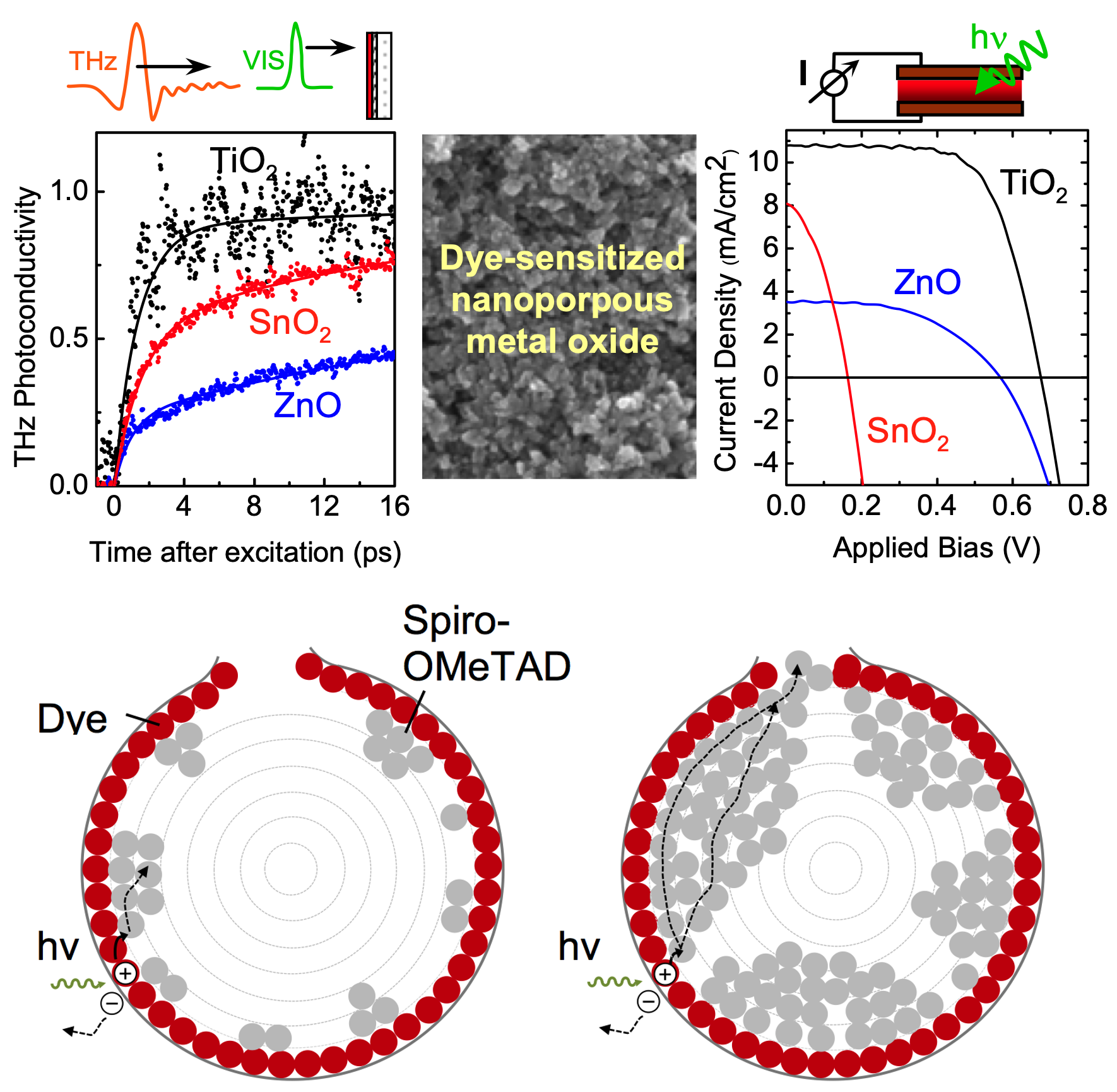
Dye-sensitized solar cells (DSCs) contain a monolayer of
light-absorbing dye (the sensitizer) adsorbed to the surface
of a mesoporous electron-transporting metal oxide, usually
fabricated from sintered nanoparticles. Light absorption promotes an
electron to the dye excited state, with subsequent electron transfer
into, and transport through the metal-oxide to the collection
point. The dye is also in contact with hole-transporter which
regenerates the oxidized dye molecules and completes the
circuit. Using ultrafast spectroscopic techniques we are able to
probe individually the processes leading from light absorption to
photocurrent generation. From such measurements we assess how
performance is affected by certain changes in material system
design, e.g. electrode composition and nanostructure, pore filing,
light-soaking effects or inclusion of interlayers and additives.
See e.g.: J. Phys. Chem. C, 119 (2015), p. 9159. Adv. Func. Mater.,24 (2014), p. 668. J. Phys. Chem. C, 117 (2013), p. 668. Energy Environm. Sci., 5 (2012), p. 9566. ACS Nano, 5 (2011), p. 5158. |
|
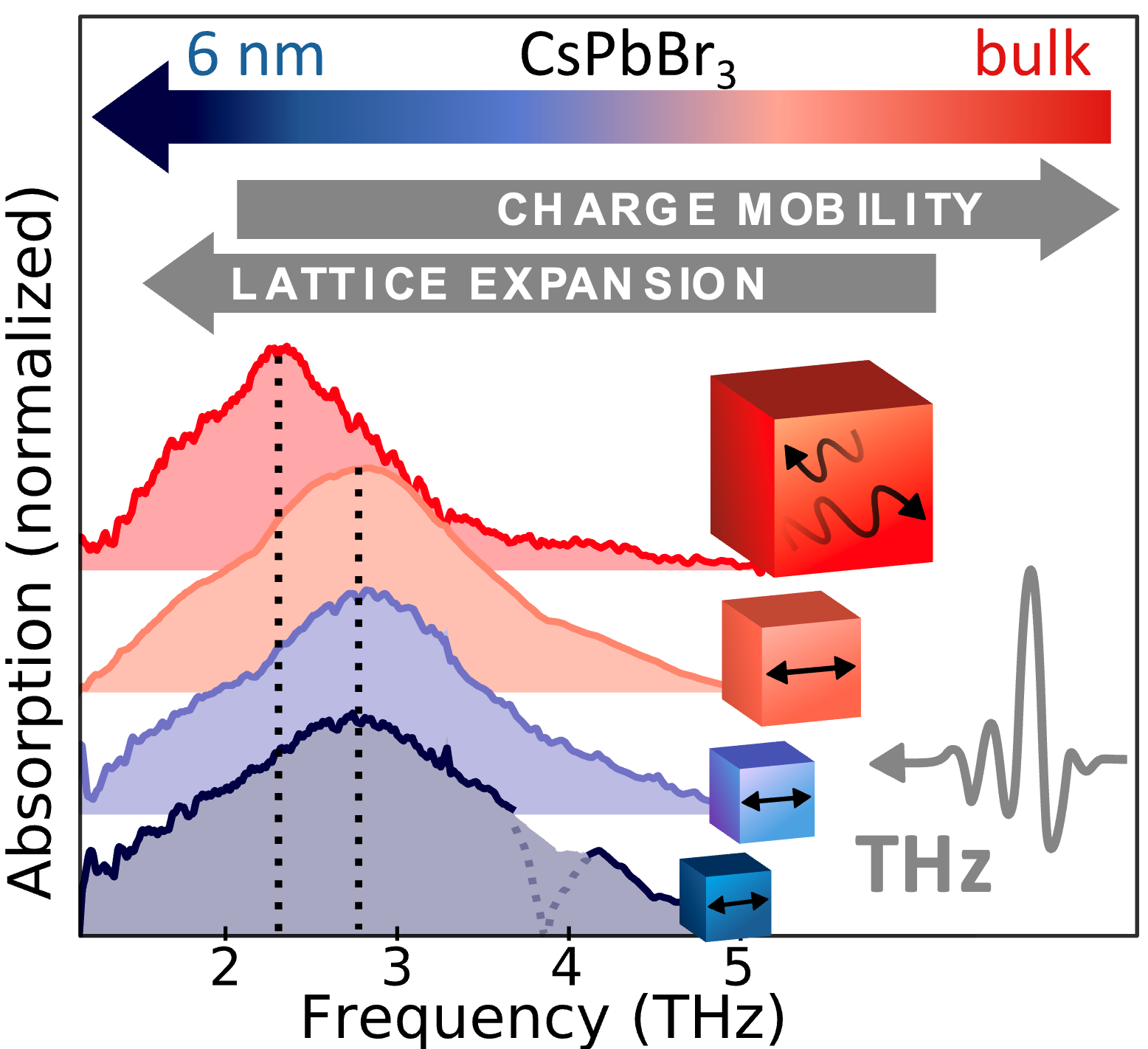
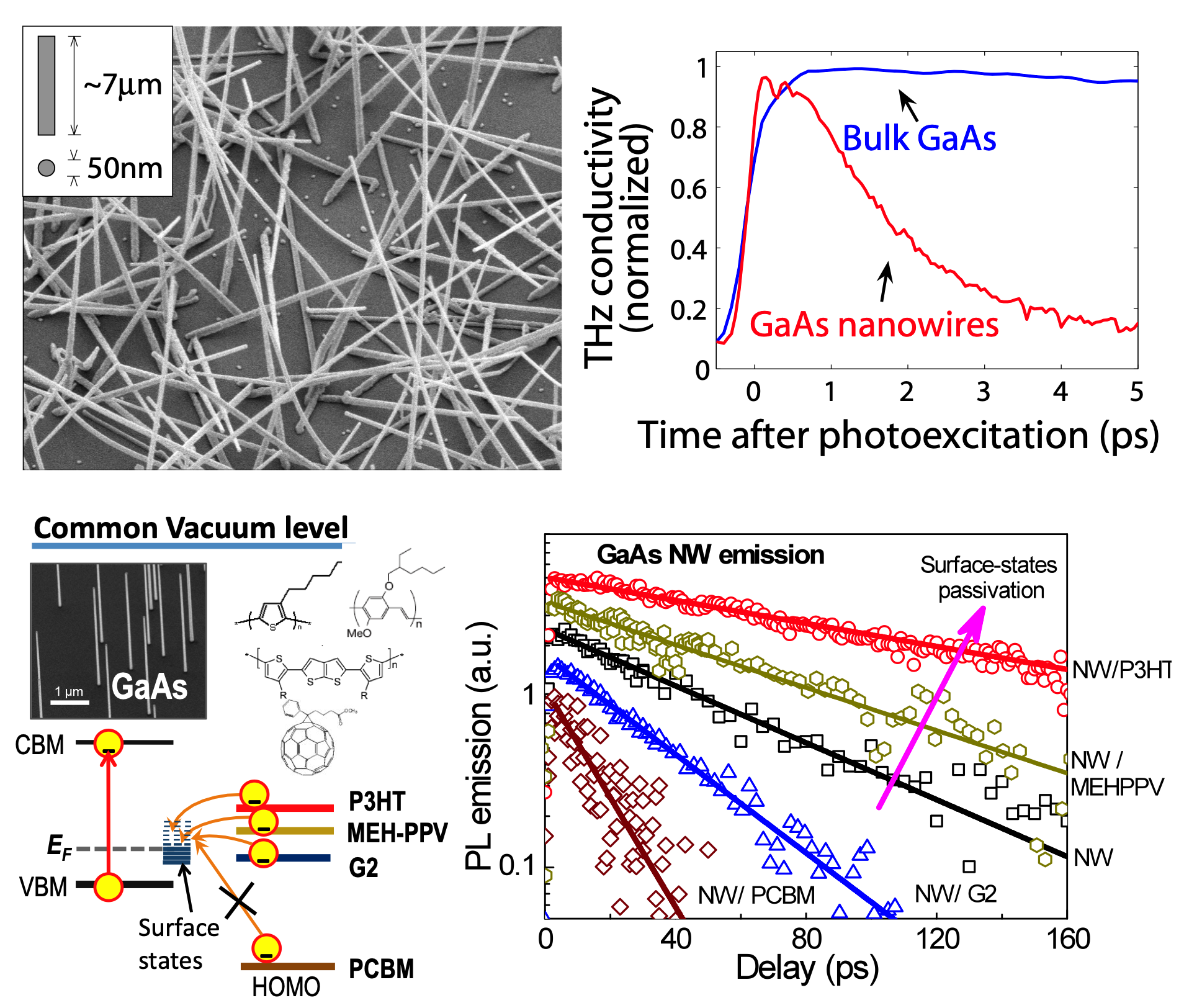
Inorganic semiconductor nanowires show tremendous potential as
ultrafast switching devices and ultrafast optoelectronic devices
such as fast photodetectors or modulators for use in very high
bandwidth communications systems. We have investigated the ultrafast
charge carrier dynamics in such nanowires using a variety of
all-optical non-contact techniques, such as photoluminescence
up-conversion and optical-pump terahertz-probe transient
spectroscopy. We explore a variety of scientific aspects, such as surface plasmons, doping,
surface defects, overcoating with organic semiconductors.
See e.g.: Science 368 (2020), p.510. Nano Lett. 18 (2018), p.3703. ACS Nano 10 (2016), p.4219 Nano Lett. 15 (2015), p.1336 Nano Lett. 14 (2014), p.5989 Nano Lett. 13 (2013), p.4280 Nano Lett. 12 (2012), p.6293 Nano Lett. 12 (2012), p.5325 Nano Lett. 12 (2012), p.4600. |
|
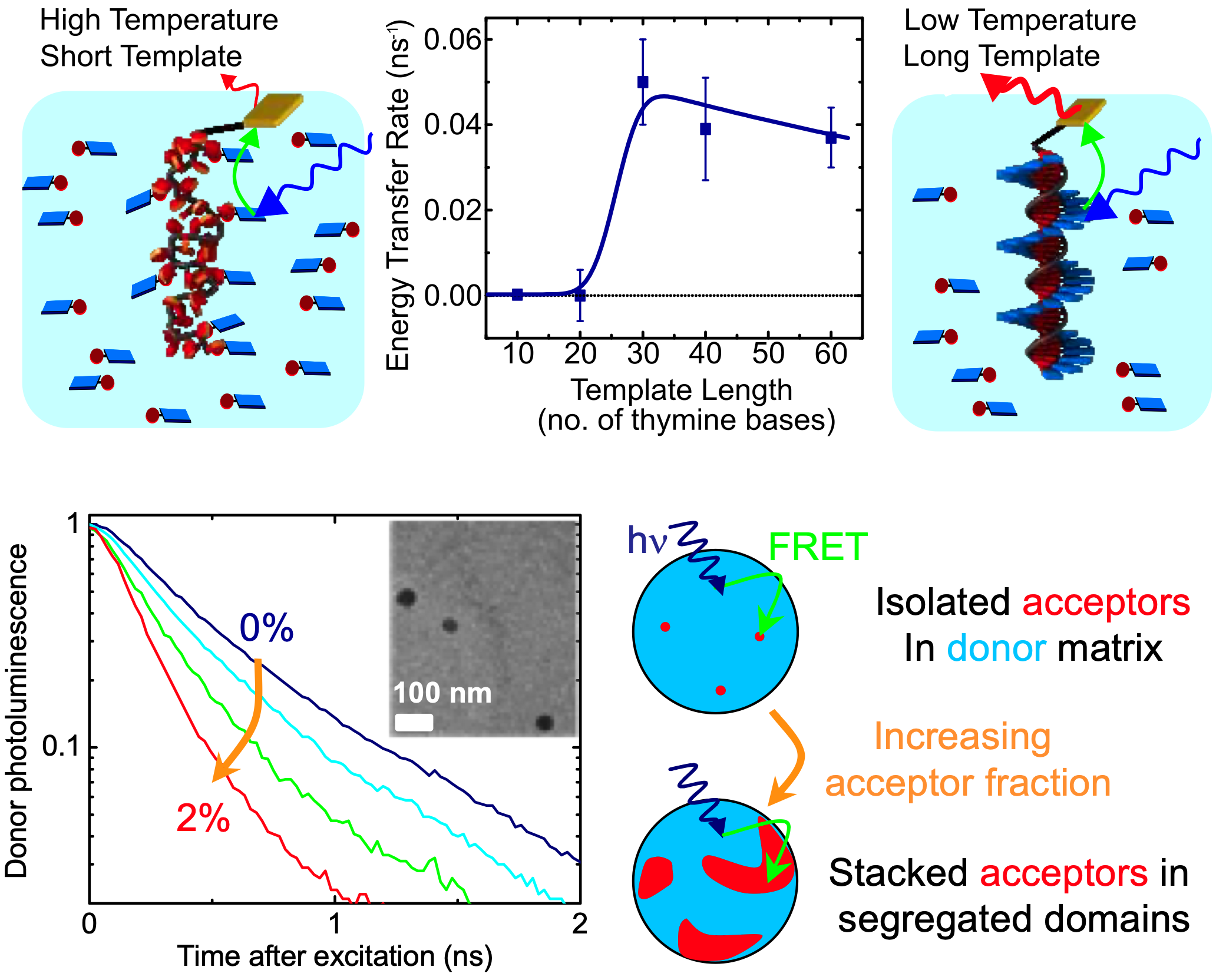
Self-assembly has fast become a staple tool used in the control of
complex supramolecular systems with applications in biological sensing. When governed by molecular
recognition, self-assembly can produce systems from solution with
specific architectures and properties that are determined by
intermolecular interactions such as hydrogen bonding, hydrophobic,
hydrophilic, and pi-pi interactions. We investigate how the
arrangement of chromophores in such supramolecular architectures
affects the transfer of photoexcitations in a variety of systems, as for
example example DNA-templated assemblies of pi-stacked conjugated
chromophores and nanoparticles, and supramolecular polymers.
See: ACS Nano, 7 (2013), p. 408. ACS Nano, 6 (2012), p. 4777. J. Phys. Chem. C, 115 (2011), p. 10550. Chem. Commun., 47 (2011), p. 884. J. Am. Chem. Soc., 131 (2009), p. 17696. |
![]() L. M. Herz, P. Nellist, M. B. Johnston, H. J. Snaith, K. Mc Kenna Tailoring structure–property relationships in metal halide perovskites at an atomistic level, EPSRC grant APP30818 (1 March 2026 – 31 Aug 2029)
L. M. Herz, P. Nellist, M. B. Johnston, H. J. Snaith, K. Mc Kenna Tailoring structure–property relationships in metal halide perovskites at an atomistic level, EPSRC grant APP30818 (1 March 2026 – 31 Aug 2029)
![]() M.B. Johnston, H. Kraus, L.M. Herz, E. McCormack, P. Huggard Integrated THz time-domain devices for real-time hyperspectral tomography, EPSRC grant APP57181 (1 June 2025 – 31 May 2028)
M.B. Johnston, H. Kraus, L.M. Herz, E. McCormack, P. Huggard Integrated THz time-domain devices for real-time hyperspectral tomography, EPSRC grant APP57181 (1 June 2025 – 31 May 2028)
![]() L. M. Herz, Mastering charge-lattice interactions in novel semiconductors for renewable energy applications, EPSRC grant EP/Y014952/1 (1 July 2024 – 30 June 2029)
L. M. Herz, Mastering charge-lattice interactions in novel semiconductors for renewable energy applications, EPSRC grant EP/Y014952/1 (1 July 2024 – 30 June 2029)
![]() H. J. Snaith, L. M. Herz, S. Islam, M. B. Johnston, M. Rosseinsky,
M. Filip, I. McCulloch, N. Noel, Advanced Device Concepts for Next-Generation Photovoltaics, EPSRC grant EP/X038777/1 (1 Oct 2023 – 30 Sept 2028)
H. J. Snaith, L. M. Herz, S. Islam, M. B. Johnston, M. Rosseinsky,
M. Filip, I. McCulloch, N. Noel, Advanced Device Concepts for Next-Generation Photovoltaics, EPSRC grant EP/X038777/1 (1 Oct 2023 – 30 Sept 2028)
![]() L. M. Herz, M. B. Johnston, N. Noel, Exploring intrinsic quantum confinement in metal halide perovskites, Leverhulme Trust Research Grant RPG-2022-272 (1 Aug 2023 – 1 Apr 2027)
L. M. Herz, M. B. Johnston, N. Noel, Exploring intrinsic quantum confinement in metal halide perovskites, Leverhulme Trust Research Grant RPG-2022-272 (1 Aug 2023 – 1 Apr 2027)
![]() M. B. Johnston, H. Kraus, L. M. Herz, Ultrafast Terahertz Polarimetry Enabled by Semiconductor Nanowire Sensors, EPSRC grant EP/W018489/1 (1 Sept 2022 – 31 Aug 2026)
M. B. Johnston, H. Kraus, L. M. Herz, Ultrafast Terahertz Polarimetry Enabled by Semiconductor Nanowire Sensors, EPSRC grant EP/W018489/1 (1 Sept 2022 – 31 Aug 2026)